Dear deLeon,
A few comments -
(1) the answer may depend on yr product/process
(2) Generic E.coli is not a pathogen.
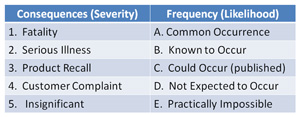
(3) the interpretations of individual entries in "likelihood/Severity" risk matrices are inevitably subjective, ie the opinion of Mr.A may not (justifiably) agree with Mr.B. Regardless there are a few entries where most people will usually agree, eg LL, HH but 25-50%(?) is undoubtedly a greyish area, and hotly debated in some places. From an auditorial POV, if ever challenged (which hasn't happened yet) i (would) say my matrix is based on a "published" one (there are sooo many 5x5s and other variations).
(3) A few detailed, published, relative risk levels of pathogens exist, mostly from memory ex-USA. 1-2 are mentioned, linked on this forum (somewhere). But I suspect the vast majority of users simple allocate severity 5/ maximum to all listed pathogens. Never had that challenged so far. The truth is that in respect to absolute potency the risk may clearly vary but I doubt that auditors expect such knowledge to be displayed to them. 
The "malleable" parameter as far as the risk calculation is concerned is, IMO, the Likelihood, interpretations for this can radically change the result, eg referenced to where ? - eg the specific step or the consumer ? Both opinions can be found in published hazard analyses.
(4) Regarding yr specific queries, it may help to find published examples of (a) yr choice of approach to hazard analysis (there are a legion), (b) yr specific hazard (this may take some effort). Or as per yr post, label it as a prerequisite and avoid the decision altogether.
Rgds / Charles.C