- Home
- Sponsors
- Forums
- Members ˅
- Resources ˅
- Files
- FAQ ˅
- Jobs
-
Webinars ˅
- Upcoming Food Safety Fridays
- Upcoming Hot Topics from Sponsors
- Recorded Food Safety Fridays
- Recorded Food Safety Essentials
- Recorded Hot Topics from Sponsors
- Food Safety Live 2013
- Food Safety Live 2014
- Food Safety Live 2015
- Food Safety Live 2016
- Food Safety Live 2017
- Food Safety Live 2018
- Food Safety Live 2019
- Food Safety Live 2020
- Food Safety Live 2021
- Training ˅
- Links
- Store ˅
- More
Advertisement
Featured Implementation Packages
-
ISO 22000 Food Safety Management System - Food Manufacturers Edition - 2018
This is an ideal package for Food Manufacturers looking to meet International Fo... more
-
SQF (& FSMA) Implementation Package for Food Manufacturers - Edition 9 (2023 Update)
This comprehensive documentation package is available for immediate download and... more
SQF from Scratch: 2.3.1 Product Development and Realization
Aug 30 2020 05:22 PM | Simon
SQF SQF 2.3.1 Product Realization
2.3.1 Product Development and Realization
Well reader, maybe this is a new role for you, maybe it’s one you’ve played at your company for a long time, but I hope that you’ll find it rewarding.
No, I’m not referring to SQF practitioner, I’m referring to the role of wet blanket technical resource for new product development activities!
The food industry can sometimes be challenging not because of the work, but because everyone knows a little something about food! After all, everyone has intimately studied and investigated their foods by eating several times a day since birth! We’re as much experts at understanding how to fix hunger as we are at growing our own hair. It comes naturally and everyone a wealth of experience to draw on.
Thus, the day comes when entrepreneurial folks say to themselves, I know how to make tasty things, let’s sell them! Unfortunately, while starting a business is hard anyway, with food there’s an extra hitch when people get sick.
Dr. Powell over at Barfblog summarizes the risk of food as a business well:
“I’ve yet to see divine intervention as a cause of foodborne illness. Instead, illnesses and outbreaks are frighteningly consistent in their underlying causes: a culmination of a small series of mistakes that, over time, results in illness and death.”
SQF recognizes that some of those small mistakes are a result of moving forward with the mass distribution of food products using the same risk evaluation as having neighbors over for a barbecue. Not only does mass distribution make illness a public health numbers game instead of a personal risk assessment, but our intimacy with food lends itself to confidence, not expertise. Just like we cannot predict the behavior of the ocean by studying a single water molecule, jumping into food based on personal kitchen experience can lead to missing details that make products dangerous (or just bad!).
- Let’s just put our tasty beverage in a can! There’s probably no extra requirements to address botulism.
- This never made me sick, so there’s no way it could hurt anyone else.
- We just sell shrimp, why do I need to know what a HACCP plan is?
- We sourced the best ingredients possible, so we covered all the potential contamination risks, right?
- I just make cheese, I’m not a microbiologist.
- It’s got alcohol in it, so that makes it safe to drink.
So speak quietly, ask smart questions, and let’s carefully rain on this parade!
The code:
2.3.1 Product Development and Realization
2.3.1.1 The methods and responsibility for designing, developing and converting product concepts to commercial realization shall be documented and implemented.
2.3.1.2 Product formulation, manufacturing process and the fulfillment of product requirements shall be validated by site trials, shelf life trials and product testing.
2.3.1.3 Shelf life trials where necessary shall be conducted to establish and validate a product’s:
i. Handling, storage requirements including the establishment of “use by” or “best before dates”;
ii. Microbiological criteria; and
iii. Consumer preparation, storage and handling requirements.
2.3.1.4 A food safety plan shall be validated and verified for each new product and its associated process through conversion to commercial production and distribution, or where a change to ingredients, process, or packaging occurs that may impact food safety.
2.3.1.5 Records of all product design, process development, shelf life trials, and approvals shall be maintained.
What’s the point? How is this making our product safer?
The examples linked above are all too common. Before jumping in, business owners must recognize that they have liability and a public health responsibility to take reasonable precautions to prevent foodborne illness. By using this portion of the code to make sure that food safety was part of the product development process, companies can be proactive in ensuring new products are safe.
Businesses only exist when there are consistent customers. It’s both bad planning and bad form to use them as guinea pigs.
Thankfully, resources like government food safety agencies, colleges and universities, and groups like IFSQN have created a library of information to help the food entrepreneur anticipate risk.
What am I being asked to do?
2.3.1.1 The methods and responsibility for designing, developing and converting product concepts to commercial realization shall be documented and implemented.
This particular language is going to be repeated over and over again in the code in the same format:
“The methods and responsibility for [SQF code portion] shall be documented and implemented.”
This is a throwback to the food safety management system. The “methods and responsibility” is the document on hand that describes what the company intends to do and who is going to do it. It will describe what product development looks like at that specific facility and which individuals will make sure the process is followed and documented (SQF practitioner or designee typically).
Key words: the methods should be “documented”, the policy exists, and “implemented”, evidence/documentation is available to demonstrate it is being followed.
How should this program be organized? Any way that makes sense for the facility and details when different people are involved in development is fine, but the point of this article series is to provide advice for meeting the code in a direct and audit-ready manner.
The order of these requirements is really a bit scrambled, because there is already a system in place that the entire food safety management system revolves around. The sole purpose of which is to assess the risk a product might pose and take steps to address and mitigate those risks.
2.3.1.4 A food safety plan shall be validated and verified for each new product and its associated process through conversion to commercial production and distribution, or where a change to ingredients, process, or packaging occurs that may impact food safety.
This article series still hasn’t covered 2.4.3, but it’s really the first thing that should be done! Following a CODEX or HARPC approach to risk assessment for a new product in the same way that one would an existing product, all of the necessary documentation will be covered. There will be:
- A thorough risk assessment of any novel ingredients or processes in the hazard analysis
- Validation any new manufacturing/processing steps for the conditions of use (so both the R&D lab, pilot batch, and actual plant conditions)
- A review of consumer use conditions and what hazards would be presented by them (e.g. shelf life, ready-to-eat, cold supply chain)
- Microbiological criteria used for verification of microbial controls
- Documented and organized testing data used to design and develop the new product and any testing specifications
- Approval from the food safety team when they sign off on the new plan revision
Once a food safety plan is completed for a new product, the typical HACCP/HARPC hazard analysis and plan does everything that 2.3.1 requires. In some ways this portion of the code is redundant because if a product is within the scope of certification, it should have a plan under 2.4.3. However, there’s one specific piece of the code here that often trips suppliers up.
2.3.1.2 Product formulation, manufacturing process and the fulfillment of product requirements shall be validated by site trials, shelf life trials and product testing.
2.3.1.3 Shelf life trials where necessary shall be conducted to establish and validate a product’s:
i. Handling, storage requirements including the establishment of “use by” or “best before dates”;
ii. Microbiological criteria; and
iii. Consumer preparation, storage and handling requirements.

Unfortunately, a brief search of IFSQN on “shelf life trial” quickly demonstrates that this article will not be able to help define what sort of testing is necessary for a specific product any more than it could describe what hazards would be present in all products. Shelf life is a complicated determination that crosses food safety with food quality goals, local legislation, supplier agreements, and consumer perception.
However, without knowing the specific details we can still use a hazard analysis approach to document our decision-making process behind the shelf life we are using for our products, and thus meet the requirement.
The auditor isn’t on site to agree with the documented justification (assuming it’s compliant from a regulatory standpoint and somewhat reasonable), but to make sure it was carefully considered and tested when appropriate. Here’s a simple example of a form that could be used to evaluate shelf life for a new product:
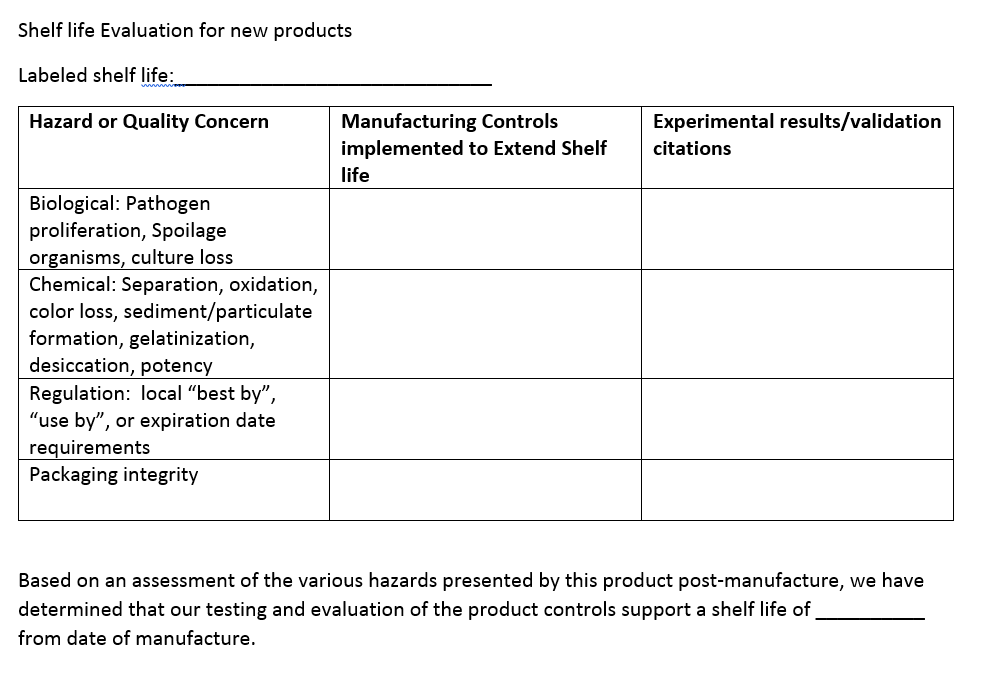
The key is to still perform an evaluation and document why they should be treated similarly. Don’t let the marketing team keep the same shelf life on the limeade if they also decided it should be fresh-squeezed and not pasteurized like the lemonade, the two products are no longer similar.
How will this be audited?
Step one is simple: the auditor will straight up ask if there have been any new products introduced to the market since the last audit.
Keep in mind that per the code, this includes:
“a change to ingredients, process, or packaging occurs that may impact food safety.”
While many companies launch new flavors each year, making the changes obvious, others may only change raw material suppliers, packaging, or configurations (e.g. introducing a larger container). Some of these changes may happen in the background of the food safety plan, and it’s helpful to document them via a change management process that can support the requirements of 2.1.3. I like to use a generic change management form to cover the management review portion as well as keep track of any activities that should be red flagged for review.
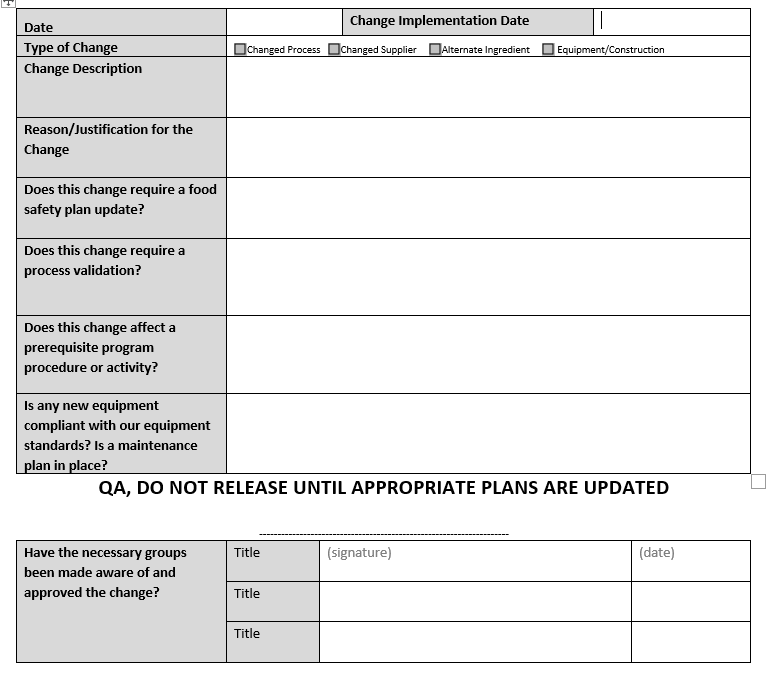
The big auditing red flag is finding a product that was introduced without any record of a development process in place. It happens. Unfortunately, while an up to date food safety plan is a good record that everything was done correctly, it doesn’t always prove that it was done prior to launch. Some sort of consolidation of the documentation that happened during development can put a gold star next to this requirement. The journal the R&D team kept about the project, a project management chart or file, a draft food safety plan for the product dated before launch, or even just a dedicated folder space where all of the data was organized can act as additional proof the site followed the methods outlined in the policy document.
2.3.1 is there to make sure that we take the proper care to bring things to market and prevent the use of customers as an experimental group. It’s part of what makes the SQF certification worth something. When sourcing from SQF suppliers, there’s an assurance that they won’t send out new products or product changes without properly vetting them first.
Have you ever received an inferior product from a supplier because of a change on their end? Maybe they reduced the amount of material used or changed a process, then shipped it out assuming it wouldn’t impact the customer. An IQF fruit provider sending a ½” dice vs. a 3/8” dice, a film supplier who switches from extruded to blown LDPE, or a corrugate supplier that changes to a new adhesive?
If companies rely on customers to determine whether new products are still safe and effective, customers will evaluate the business, not the product.
Author Biography:

Austin Bouck is a food safety consultant and manufacturing supervisor in Oregon, USA. You can find more food safety resources and discussion on his website, Fur, Farm, and Fork, as well as contact information for consulting services.









0 Comments