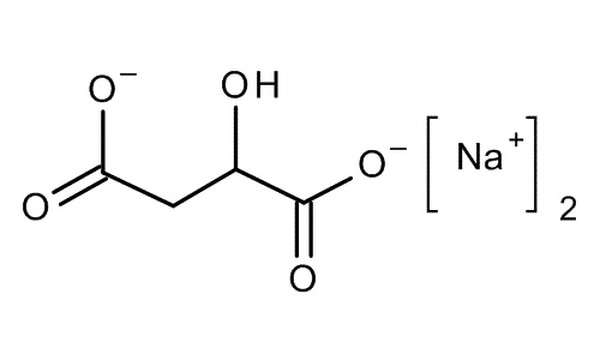
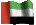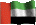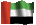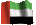I've just realised the bottom chemical structure is flipped from the top. Unfortunately it's been a long time since I've had a chemical drawing programme so I had to get these off the internet. For people who aren't used to "reading" diagrams like this, both structures are the same apart from one has one of the acid groups converted to a salt with sodium and the other has both. They're just rotated.
For complete chemistry nerds, to add a bit more complexity... yes this would be a racemic mixture when you buy it off the shelf (that's what the DL stands for) as the OH group would make it chiral.
What is fascinating is that while malic acid is natural, having it as a racemic mixture isn't (it's only L in nature) and that's where you get into the real iddy biddy detail on chemical interactions with bodies and unexpected outcomes. So it might seem weird that people may question the safety of something pretty ubiquitous in nature (malic acid is abundant in apples and these are just the salts of malic acid) but it's perhaps not quite the same. Try explaining that to an online "all chemicals are bad" conspiracy theorist...
"well no, and everything is a chemical anyway... but because carbon can have four bonds this creates the opportunity for chemicals with identical properties outside the body which don't always react the same way with us because they may be hydrogen bonding to our own, complex molecules. And sometimes our bodies recognise mirror image chemicals differently because that temporary bonding with our own chiral molecules makes it effectively diastereoisomeric..."

Edited by GMO, Today, 09:12 AM.