- Home
- Sponsors
- Forums
- Members ˅
- Resources ˅
- Files
- FAQ ˅
- Jobs
-
Webinars ˅
- Upcoming Food Safety Fridays
- Upcoming Hot Topics from Sponsors
- Recorded Food Safety Fridays
- Recorded Food Safety Essentials
- Recorded Hot Topics from Sponsors
- Food Safety Live 2013
- Food Safety Live 2014
- Food Safety Live 2015
- Food Safety Live 2016
- Food Safety Live 2017
- Food Safety Live 2018
- Food Safety Live 2019
- Food Safety Live 2020
- Food Safety Live 2021
- Training ˅
- Links
- Store ˅
- More
Advertisement
Featured Implementation Packages
-
BRCGS Agents and Brokers Product Safety Management System - Issue 3
This is an ideal package for Agents and Brokers of Food and Packaging Materials... more
-
SQF Implementation Package for Storage & Distribution Operations - Edition 9
This comprehensive documentation package is available for immediate download, ca... more
Latest Articles


Outside, In: Mapping Food Processing’s Pest Hot Spots
Apr 29 2020 01:35 PM | Simon in
Due to their abundance of food, shelter and water, food processing facilities are an ideal place for pests to live and breed. Unfortunately, the presence of pests in your facility threatens the safety and quality of your product. These pesky intruders can slow your operations by contaminating food, causing equipment damage and potential disease transmission.
Knowing your site’s pest hot spots and taking a proactive approach to pest management can help prevent pests from chewing away at your reputation and bottom line.
An Integrated Pest Management (IPM) program is one of the most effective ways to be proactive with your program. Not only does this help defend the facility against pests, an IPM approach ensures you are meeting essential government, industry and audit requirements. Knowing the hot spots and responding appropriately around your facility is critical to pest prevention.
Read story → 0 comments






SQF from Scratch: 2.1.5 Crisis Management Planning
Apr 14 2020 05:10 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 1 comments





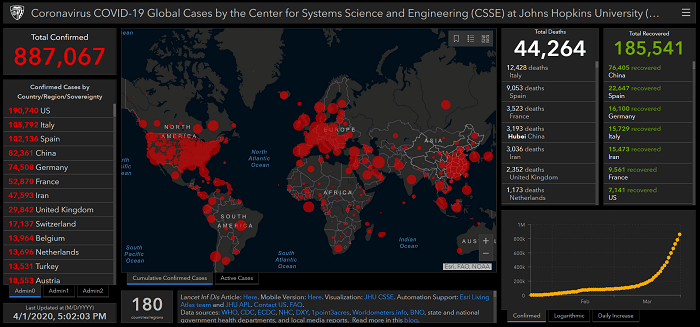
Managing the risks of COVID-19 in the Food Industry
Apr 01 2020 12:45 PM | Simon in
At the end of 2019 as people were making their New Year’s resolutions and plans for 2020; nobody imagined the equilibrium of life would be affected so dramatically by a virus outbreak that began in Wuhan, a place in Hubei province, China.
What started as an epidemic in China, has now become a truly global pandemic. WHO declared it a pandemic on March 11 and so far, it has affected more than 200 countries. Currently the pandemic is still spreading and the best visual of this is presented by John Hopkins University Covid-19 dashboard, which collates information from national and international health authorities.
Read story → 0 comments





SQF from Scratch: 2.1.4 Complaint Management
Mar 08 2020 02:39 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 0 comments






SQF from Scratch: 2.1.3 Management Review
Jan 30 2020 06:45 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 1 comments





SQF from Scratch: 2.1.2 Management Responsibility
Jan 12 2020 06:15 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Read story → 5 comments






SQF from Scratch: 2.1 Management Commitment, 2.1.1 Food Safety Policy
Jan 10 2020 07:02 PM | FurFarmandFork in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Read story → 9 comments






Particle Contamination in Compressed Air: Choosing the right analytical method per ISO 8573
Nov 28 2019 07:49 PM | Simon in
By Jenny Palkowitsh and Stephanie Suarez of Trace Analytics, LLC
Food and beverage manufacturers must carefully monitor their compressed air systems to ensure that contamination will not impact their end products. Particle contamination is of great concern to food and beverage manufacturers, and is discussed at length in the ISO 8573 standard. ISO 8573 specifies purity classes with varying limits of particle contamination. Manufacturers can use these classes along with a risk assessment to determine which purity classes will meet their specific needs. Once this is decided, part 4 of the standard can be used to determine which method of particle analysis is most appropriate for their use. Depending on the purity levels required for the system, either gravimetry, microscopy, laser particle counters (LPC), or a scanning electron microscope (SEM) can be employed. This article will cover the differences between these analytical methods and help manufacturers determine which type of analysis is appropriate to meet their standards and to ensure product safety.
Read story → 0 comments






Working towards smarter food safety objectives
Oct 07 2019 10:08 AM | Simon in
My name is Jean-Guy Cormier, P.Eng., Lead Auditor. I am a professional engineer and a management system Lead Auditor for 30 years, the last 10 years with DNV GL Business Assurance, one of the top certification bodies in the world. As a professional auditor, I have done over a thousand audits in food safety, quality management, environmental and health and safety management system.
This article discusses the establishment of measurable targets to help management teams to focus their resources within the required areas and timeframes. Also, it is a platform for continuous improvement initiatives either at the plant-level or process-specific levels.
This article addresses three fundamental questions:
- How and why does management establish measurable quality and food safety objectives?
- How does management establish continuous improvement initiatives that are directly linked to measurable objectives? And
- How and why management should establish proactive food safety objectives?
Unfortunately, too many companies still struggle because of several factors, such as lack of knowledge and understanding, not fully grasping the benefits and added value, lack of discipline and structured approach.
Read story → 8 comments





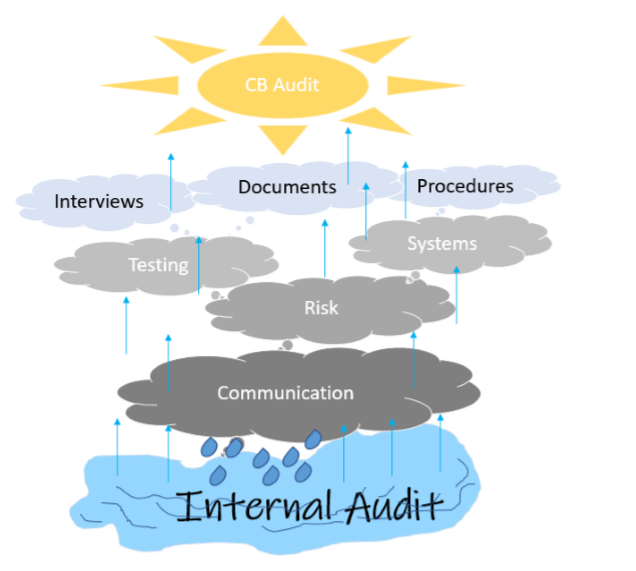
Ineffective Internal Audit: Underlying Causes
Sep 09 2019 02:36 PM | Simon in
My name is Jean-Guy Cormier, P. Eng. Lead Auditor. I am a professional engineer providing consulting and auditing services for almost 30 years. As a professional auditor, I have done over a thousand audits against food safety, quality management, environmental and health and safety management systems. In the past 10 years, I have done over 250 FSSC audits in the food and packaging industry. I have been working as Lead Auditor for DNV GL - Business Assurance, one of the leading global certification bodies, for 10 years.
After so many years, one observation that continues to linger is ineffective internal audit process. Almost every audit that I have participated in, there have been findings against requirements of internal audits. Arguably, some of the underlying causes, why internal audit process is ineffective are:
- inadequate training and ongoing professional development;
- poor preparation;
- poor utilization of adequate tools;
- not having the abilities to communicate in writing and/or verbally;
- Poor skill sets to collect samples of observations and supporting evidences.
Read story → 7 comments






How Rodents are Threatening Your Food Safety Procedures
Aug 05 2019 06:28 PM | Simon in
Twenty percent of the world’s food supply is believed to be contaminated by rodents. These pests can carry various pathogens around facilities and can even transmit harmful diseases. Rodents are known to cause severe property damage with their strong jaws and burrowing skills. Unfortunately, food processing facilities offer the ideal habitat for rodents with access to food and water sources, potential entry points and hiding places.
Read story → 1 comments












