- Home
- Sponsors
- Forums
- Members ˅
- Resources ˅
- Files
- FAQ ˅
- Jobs
-
Webinars ˅
- Upcoming Food Safety Fridays
- Upcoming Hot Topics from Sponsors
- Recorded Food Safety Fridays
- Recorded Food Safety Essentials
- Recorded Hot Topics from Sponsors
- Food Safety Live 2013
- Food Safety Live 2014
- Food Safety Live 2015
- Food Safety Live 2016
- Food Safety Live 2017
- Food Safety Live 2018
- Food Safety Live 2019
- Food Safety Live 2020
- Food Safety Live 2021
- Training ˅
- Links
- Store ˅
- More
Advertisement
Featured Implementation Packages
-
SQF Implementation Package for Pre-processing of Plant Products - Edition 9
This comprehensive documentation package is available for immediate download and... more
-
BRCGS Agents and Brokers Product Safety Management System - Issue 3
This is an ideal package for Agents and Brokers of Food and Packaging Materials... more
Latest Articles


METTLER TOLEDO Helps Manufacturers Cut Costs and Risk with Test Before You Invest Programme
May 07 2025 10:54 AM | Simon in
With operational costs rising and production line efficiency under pressure, manufacturers need to be confident that any new equipment investment will deliver value. That’s why METTLER TOLEDO Product Inspection is spotlighting its Test Before You Invest programme - a global service designed to help food, pharmaceutical and packaging manufacturers make informed product inspection choices through hands-on testing, real performance data and expert support.
Read story → 0 comments






From Detection to Defense: Mastering Rodent Control in Your Facility
Oct 16 2023 10:27 AM | Simon in
As a business owner, you have a multitude of challenges to contend with, but one of the most insidious and damaging threats to your operations might be lurking in the shadows: rodents. These stealthy intruders not only jeopardize the integrity of your property but can also carry diseases that pose serious risks to your employees and public health.
While post-pandemic activities have helped reduce the amount of public rodent sightings, their threat to public health hasn’t decreased. In fact, these filthy pests can spread dozens of harmful diseases directly and indirectly — like salmonellosis, leptospirosis and hantavirus — in addition to contaminating food products and causing structural damage in buildings. Left untreated, rodent sightings within a commercial facility can lead to ongoing infestations and eventually, failed inspections and stalled operations — a costly blow to your bottom line.
Knowing how to spot rodent activity is essential to stopping them early.
Read story → 0 comments






The Key to Audit Preparedness in Your Food-Processing Facility
Oct 27 2022 02:48 PM | Simon in
Although the pest control portion of an audit may account for up to 20 percent of your final score, it’s not worth losing points or failing because of a preventable issue. Pest issues can cause multiple problems for your business, and not always the way you think. Certainly, they can damage or contaminate product and finished goods. They can harm or even destroy your hard-earned reputation and brand leading to loss of valuable business. Pests can affect the health and safety for your employees and make it difficult to maintain staffing levels effecting your productivity and ability to meet your customers’ needs. Staying audit-ready year-round will help you ensure you ace the pest control portion of your next inspection, whether planned or unexpected.
Read story → 1 comments






Building A Food Safety Culture
Oct 12 2022 04:52 PM | Simon in
What food safety culture means, why is it important and how to go about creating one in your business.
With rising consumer awareness, ever-changing regulatory standards and emerging threats to food supply & sanitation, the conversation for food businesses is shifting from what we make, to how we make what we make.
Read story → 0 comments






Sources of Contamination in Food Grade Compressed Air
Sep 21 2021 02:23 PM | Simon in
By Jenny Palkowitsh | Business Development Director | Trace Analytics, LLC
Dynamic, fluid, and critical, compressed air systems can be an overlooked source of contamination in food, beverage, and packaging manufacturing. Contaminated compressed air can be detrimental to end-products, ultimately affecting bottom line numbers and company reputation. Many facilities establish monitoring plans and programs but neglect a vital part of that process: testing. Compressed air testing allows users to identify and remediate contamination in the system before it affects products. Regular testing ensures that maintenance schedules and programs are adequate and working as expected. But how does contamination enter a compressed air system? There are a wide range of sources that contamination can come from including the compressor itself, the distribution piping, the intake, and regular maintenance activities. One of the benefits of regular testing is the ability to troubleshoot the sources of contamination.
Read story → 0 comments






Uncovering the Strategies to Establish Food Safety Culture in Food Service Facilities
Apr 05 2021 05:44 PM | Simon in
Food safety culture is critical to wellness of food and overall success of a restaurant's food safety control system and given the significance, Codex Alimentarius and HACCP has embraced food safety culture as a point of focus in the current revision. This article will help food service leaders in establishing food safety culture and knowing why it is necessary and how it can benefit the organization.
Read story → 1 comments






SQF Edition 9 Updates and Compressed Air Monitoring - what you need to know
Apr 05 2021 04:00 PM | Simon in
Safe Quality Foods (SQF) has released Edition 9 Codes with improvements and updates that will be implemented May 24th, 2021. According to a SafetyChain poll, just 3% of respondents were completely ready for the SQF Edition 9 changes as of January 2021 (Chuboff, 2021). Though there are a number of changes, this article will focus specifically on the compressed air requirements that have been added and updated to provide definitions and additional clarification. SQF has also defined “compressed air monitoring” in the appendix. For many, these modifications alleviate the worry of misunderstanding or misrepresenting the requirements. Edition 9 provides clarifications that are helpful to manufacturers who are trying to meet standards, maintain a level of quality, and understand the safety requirements for their compressed air systems.
Read story → 0 comments





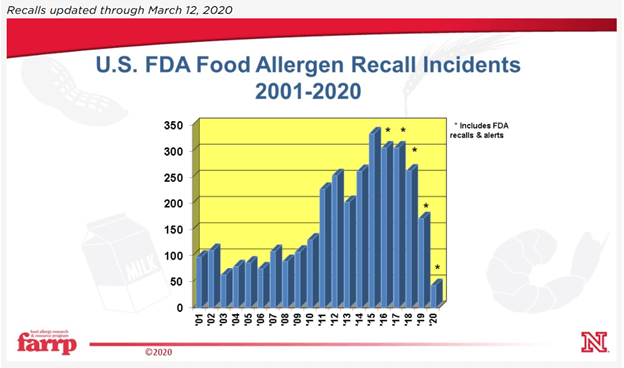
SQF from Scratch: 2.3.2.6 Finished Product Labels
Jan 26 2021 11:01 AM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 0 comments






SQF-2.3.2 Raw and Packaging Materials
Oct 26 2020 12:21 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 0 comments





SQF from Scratch: 2.3.1 Product Development and Realization
Aug 30 2020 05:22 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 0 comments





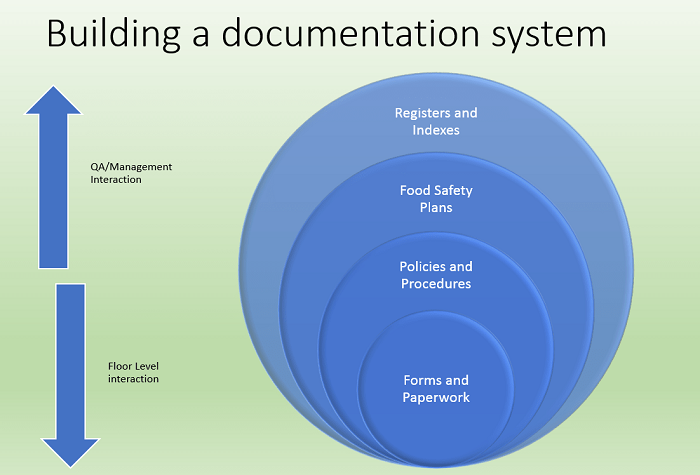
SQF from Scratch: 2.2.1 Food Safety Management System, 2.2.2 Document Control, 2.2.3 Records
Jun 09 2020 07:20 PM | Simon in
This article series is a deep dive into each individual SQF element. Not just what the code says but what’s actually being asked, how it makes our products safer, and how that element looks in practice both inside and outside the audit. Personnel new to SQF seeking implementation and those reviewing existing systems should both benefit from a methodical study of each element, and how we can truly embrace the code and share it with internal and external customers.
Remember, the goal is not “Audit ready 365”, it’s to know that our facility embraces globally recognized best practices to maintain food safety. Because of this, as we dive into each element, we must always remember the quality management system golden rule:
Never make systems to “pass audits”. Make systems that work for your company that help it make safer/higher quality products more efficiently.
Read story → 0 comments












